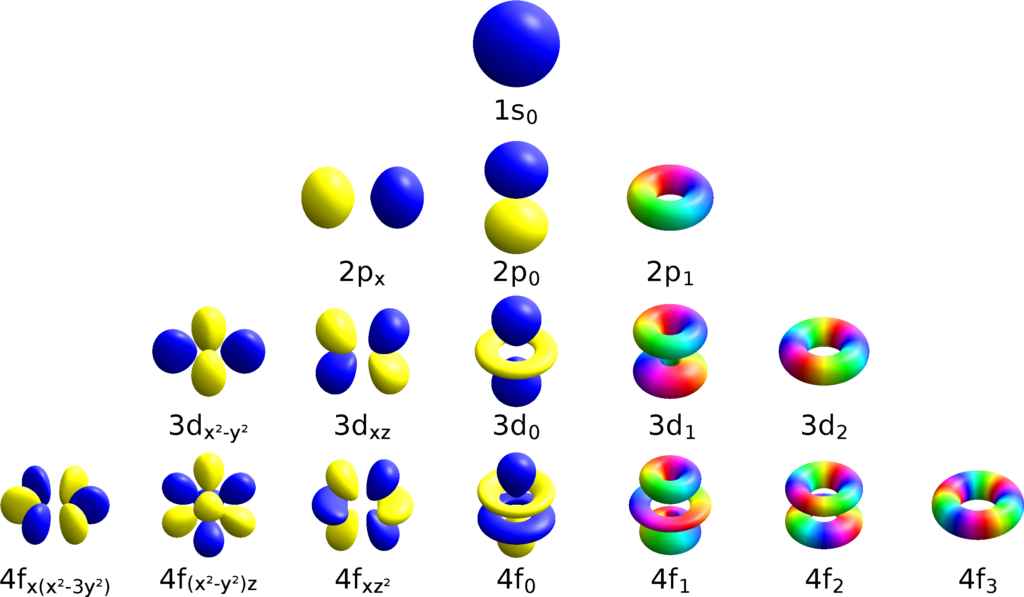What Is The Principal Quantum Number(N) For The 3D Orbital . the number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. The 3d sublevel corresponds to the principal quantum. learn about the four quantum numbers that describe the position and energy of electrons in atoms. learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. Find out the values and meanings of. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. learn how to use quantum numbers to describe the energy level, orbital type, and spin of electrons. the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom.
from chemistryskills.com
learn how to use quantum numbers to describe the energy level, orbital type, and spin of electrons. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. Find out the values and meanings of. the number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. learn about the four quantum numbers that describe the position and energy of electrons in atoms. The 3d sublevel corresponds to the principal quantum. the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom.
Quantum Numbers Chemistry Skills
What Is The Principal Quantum Number(N) For The 3D Orbital learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. Find out the values and meanings of. The 3d sublevel corresponds to the principal quantum. the number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. learn about the four quantum numbers that describe the position and energy of electrons in atoms. the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom. learn how to use quantum numbers to describe the energy level, orbital type, and spin of electrons. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons.
From mavink.com
Quantum Numbers Orbitals Chart What Is The Principal Quantum Number(N) For The 3D Orbital learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. learn about the four quantum numbers that describe the position and energy of electrons in atoms. The 3d sublevel. What Is The Principal Quantum Number(N) For The 3D Orbital.
From chemistryskills.com
Quantum Numbers Chemistry Skills What Is The Principal Quantum Number(N) For The 3D Orbital the number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. The 3d sublevel corresponds to the principal quantum. learn about the four quantum numbers that describe. What Is The Principal Quantum Number(N) For The 3D Orbital.
From www.meta-synthesis.com
Quantum Number Periodic Table Chemogenesis What Is The Principal Quantum Number(N) For The 3D Orbital The 3d sublevel corresponds to the principal quantum. learn how to use quantum numbers to describe the energy level, orbital type, and spin of electrons. the number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. learn how the principal quantum number (n) determines the average distance of. What Is The Principal Quantum Number(N) For The 3D Orbital.
From pressbooks.bccampus.ca
8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry What Is The Principal Quantum Number(N) For The 3D Orbital learn how to use quantum numbers to describe the energy level, orbital type, and spin of electrons. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. learn about the four quantum numbers that describe the position and energy of electrons in atoms. learn how. What Is The Principal Quantum Number(N) For The 3D Orbital.
From physicscatalyst.com
Quantum Numbers Chart physicscatalyst's Blog What Is The Principal Quantum Number(N) For The 3D Orbital learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. Find out the values and meanings of. the number before the orbital name (such as 2s, 3p, and so. What Is The Principal Quantum Number(N) For The 3D Orbital.
From wiringdatabaseinfo.blogspot.com
Use The Orbital Diagram For Nitrogen To Write Quantum Numbers For The What Is The Principal Quantum Number(N) For The 3D Orbital learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. learn how to use quantum numbers to describe the energy level, orbital type, and spin of electrons. learn about the four quantum numbers that describe the position and energy of electrons in atoms. Find out the. What Is The Principal Quantum Number(N) For The 3D Orbital.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is The Principal Quantum Number(N) For The 3D Orbital learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. Find out the values and meanings of. learn about the four quantum numbers that describe the position and energy of electrons in atoms. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement. What Is The Principal Quantum Number(N) For The 3D Orbital.
From byjus.com
What is the total number of orbitalsassociated with the principal What Is The Principal Quantum Number(N) For The 3D Orbital learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom. Find out the values and meanings of. learn how the principal quantum number (n) determines. What Is The Principal Quantum Number(N) For The 3D Orbital.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID2135857 What Is The Principal Quantum Number(N) For The 3D Orbital the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom. the number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. The 3d sublevel corresponds to the principal quantum. learn how the principal quantum number (n) determines. What Is The Principal Quantum Number(N) For The 3D Orbital.
From www.w3schools.blog
Quantum Numbers W3schools What Is The Principal Quantum Number(N) For The 3D Orbital learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. Find out the values and meanings of. The 3d sublevel corresponds to the principal quantum. learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. learn how to use. What Is The Principal Quantum Number(N) For The 3D Orbital.
From www.technocrazed.com
Principal quantum number n and maximum number of electrons per shell What Is The Principal Quantum Number(N) For The 3D Orbital learn how to use quantum numbers to describe the energy level, orbital type, and spin of electrons. learn about the four quantum numbers that describe the position and energy of electrons in atoms. the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom. Find out the. What Is The Principal Quantum Number(N) For The 3D Orbital.
From socratic.org
What is the difference between electron shells and electron orbitals What Is The Principal Quantum Number(N) For The 3D Orbital learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. learn about the four quantum numbers that describe the position and energy of electrons in atoms. Find out the values and meanings of. the principal quantum number (n) is one of four quantum numbers that describe. What Is The Principal Quantum Number(N) For The 3D Orbital.
From www.chegg.com
Solved you can ignore the principle quantum number n What Is The Principal Quantum Number(N) For The 3D Orbital Find out the values and meanings of. learn about the four quantum numbers that describe the position and energy of electrons in atoms. the number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. the principal quantum number (n) is one of four quantum numbers that describe the. What Is The Principal Quantum Number(N) For The 3D Orbital.
From www.youtube.com
The Principal Quantum Number (n) YouTube What Is The Principal Quantum Number(N) For The 3D Orbital learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom. learn about the four quantum numbers that describe the position and energy of electrons in. What Is The Principal Quantum Number(N) For The 3D Orbital.
From chemistrytalk.org
Electron Orbitals & Orbital Shapes ChemTalk What Is The Principal Quantum Number(N) For The 3D Orbital The 3d sublevel corresponds to the principal quantum. learn about the four quantum numbers that describe the position and energy of electrons in atoms. the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom. the number before the orbital name (such as 2s, 3p, and so. What Is The Principal Quantum Number(N) For The 3D Orbital.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin What Is The Principal Quantum Number(N) For The 3D Orbital Find out the values and meanings of. the number before the orbital name (such as 2s, 3p, and so forth) stands for the principal quantum number, n. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. learn how the principal quantum number (n) determines the. What Is The Principal Quantum Number(N) For The 3D Orbital.
From drivenheisenberg.blogspot.com
Use The Orbital Diagram For Nitrogen To Write Quantum Numbers For The What Is The Principal Quantum Number(N) For The 3D Orbital learn how the principal quantum number (n) determines the average distance of an electron from the nucleus. learn about the four quantum numbers that describe the position and energy of electrons in atoms. The 3d sublevel corresponds to the principal quantum. the principal quantum number (n) is one of four quantum numbers that describe the state of. What Is The Principal Quantum Number(N) For The 3D Orbital.
From www.chemistrylearner.com
Principal Quantum Number Definition, Determination, & Value What Is The Principal Quantum Number(N) For The 3D Orbital the principal quantum number (n) is one of four quantum numbers that describe the state of an electron in an atom. learn how to use the four quantum numbers (principal, orbital, magnetic, and spin) to describe the movement and trajectories of electrons. learn how to use quantum numbers to describe the energy level, orbital type, and spin. What Is The Principal Quantum Number(N) For The 3D Orbital.